If a Substance Is Alkaline Which Term Best Describes I
A sample of chlorine and a sample of sodium were placed in a test tube and heated over a flame. Which describes an alkaline solution.
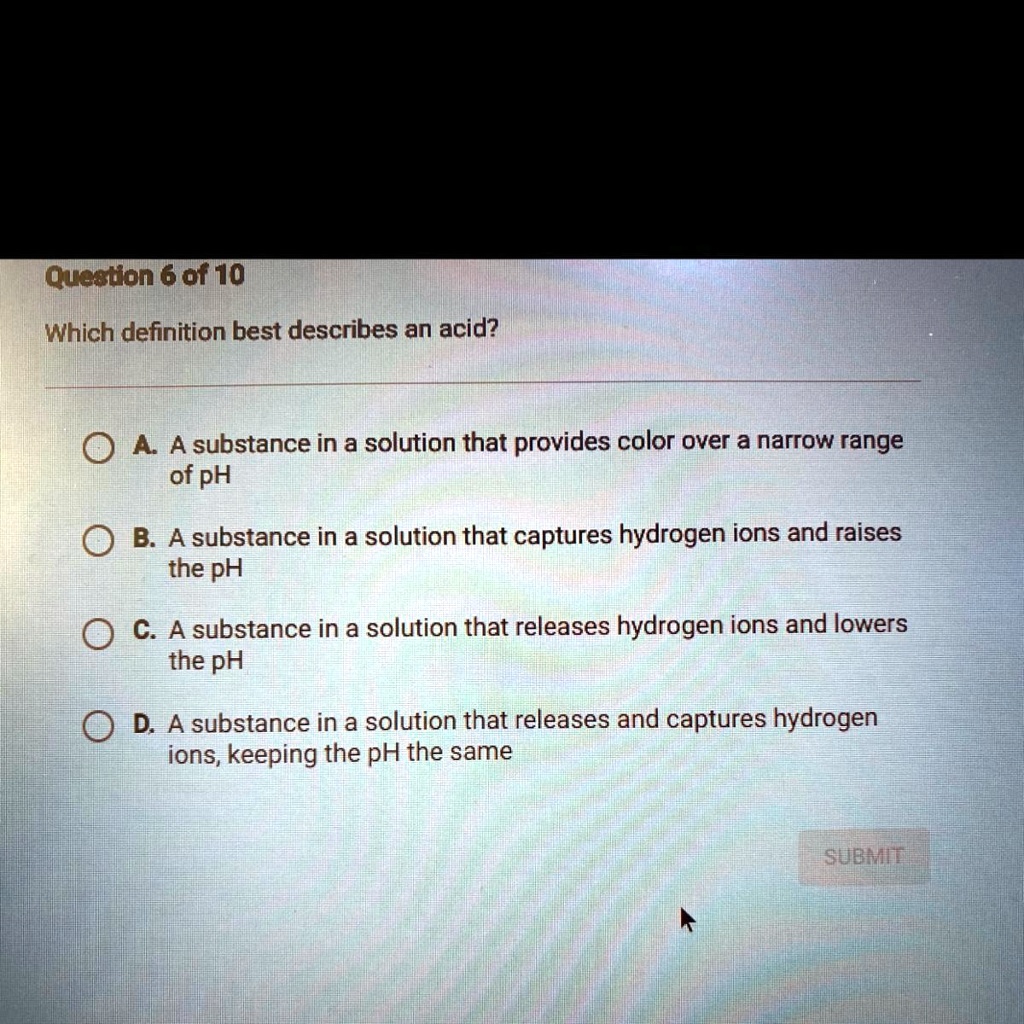
Solved Which Definition Best Describes An Acid Queeton 6 Of 10 Which Definition Best Describes An Acid 0 4 A Substance In A Solution That Provides Color Over A Narrow Range Of Ph
Any substance that has a pH higher than 7 is an alkali or alkaline substance which is also called basic.

. Alkaline is just a term used to describe a variety of substances with. A substance with a pH of 2 has a 100 times higher concentration of H ions than a substance with a pH of 3. 6 C 2020 06203120 3 Some properties of four substances A B C and D are shown in the table.
Wikipedia defines an alkali as. In order to convert from pOH to pH we can use the equation pH 14 - pOH. The probability of finding an electron at any particular point in an atom.
Therefore any basic material in this group that dissolves in water may result in an alkaline solution. Alkaline solutions are also referred to as basic as in the opposite of acidic and have a pH above 7. The table displays the physical properties of some common elements.
There are many substances that are alkaline such as soap toothpaste dock leaves and detergents. The term alkaline is derived from the metal elements of group 1 and group 2 in the periodic table of elements. Which statement best describes the pH of pure water.
B A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. A substance that does not ionize in water and consequently gives a nonconducting solution. Scientists can identify and element by looking at the structure of a single.
PH less than 7 pH equal to 7 basic acidic. The group 2 metals are called alkali earth metals. A soulution is acidic if it has more negative hydroxide ions.
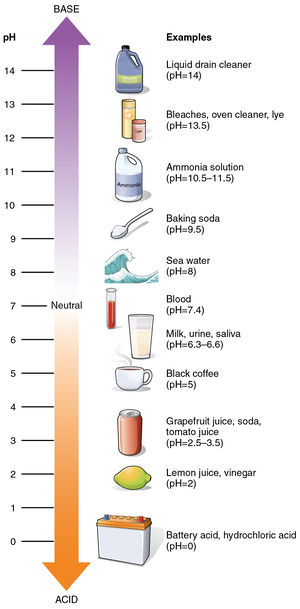
Now Ive come across the term alkaline on numerous occasions and pretty much all examples that I know of in Chemistry actually do involve the presence of an alkali often NaOH or KOH. We can find out how acidic or basic a substance is by using the pH scale. This is a term that describes the state of an environment or solution that is alkali in nature and whose pH is greater than 7.
Concept 31 Hint. Saltwater vinegar bronze air and beach sand are in which category. PH less than 7 pH equal to 7 basic acidic.
Which of the following terms is used to describe the male and female. Is it true that A substance with equal numbers of H ions and OH- ions is an alkaline solution. Most solutions are either acidic or basic also called alkaline - a substance that is neither acidic nor a basic is neutralAcids and bases are very important chemically and are found almost everywhere.
Alkaline substance that makes up the largest part of the seminal fluid. The best way to separate salt from water is with the use of. Any substance that has a pH lower.
Sodium Na and chlorine Cl combine to form sodium chloride NaCl which is commonly known as. Substances of an acidic or alkaline nature dissolve in water and or contain. Enzyme - An enzyme is a protein that acts as a catalyst in a biochemical reaction.
Concept 33 A A buffer accepts hydrogen ions when they are in excess and donates hydrogen ions when they have been depleted. Select the statement that best describes a buffer. Metals that form simple positive ions 2 in water to form hydrides and result in a pH of more than 7 are called alkaline metals.
The backbone of all protien molecules is the. Select the term best describing the series of elements. Entropy - Entropy is a measure of the disorder or randomness in a system.
Alkaline An adjective that describes a chemical that can accept a proton from another molecule or donate a pair of electrons. Equilibrium - Equilibrium occurs in reversible reactions when the forward rate of the reaction is the same as the reverse rate of the reaction. Ammonia is a compound made from the elements nitrogen and hydrogen.
Density 217 gcm3. Any substance that has a pH lower than 7 is an acid. Which of the following best describes a strong electrolyte.
The group1 elements are called alkali metals. A Li b Na c Rb d F e I 4. Search for an answer or ask Weegy.
Basic describes an alkaline solution. Which of the following terms accurately describes the energy associated with the. Group of answer choices A substance that is completely ionized in solution.
Substance strength ductility how easy it is to pull into a wire hardness conductivity of heat A weak poor hard poor B strong not ductile very hard good C very strong very good hard good D weak poor soft good Answer these questions using only the information in the table. All of the following are true regarding PH EXCEPT. Which element has the largest atomic radius.
The greater the pH number is the more basic the substance is. Concept 31 Hint 2. A d-transition metals b representative elements c metalloids d alkaline earth metals e halogens 3.
Our new pH values are then solution A 125 solution B 70 and solution C 05. Which of the following terms describes the structure that connects the uterus to the outside of the body a passage into which sperm is introduced and the canal through which a baby is born. Mn Fe Co Ni Cu.
In chemistry an alkali is a basic ionic salt of an alkali metal or alkaline earth metal. Most of waters unique properties result from the fact that water molecules _____. Which describes an alkaline solution.
Ammonia A colorless gas with a nasty smell. Alkali refers to any basic hydroxide or a salt of alkali metals or alkaline earth metals. Water molecules have a polarity which allows them to be electrically attracted to other water molecules and other polar molecules by weak chemical bonds known as _____.
The properties of the product are listed below. The term pH is an abbreviation for power of Hydrogen which is a measure of how acidic or basic a chemical solution is.

No comments for "If a Substance Is Alkaline Which Term Best Describes I"
Post a Comment